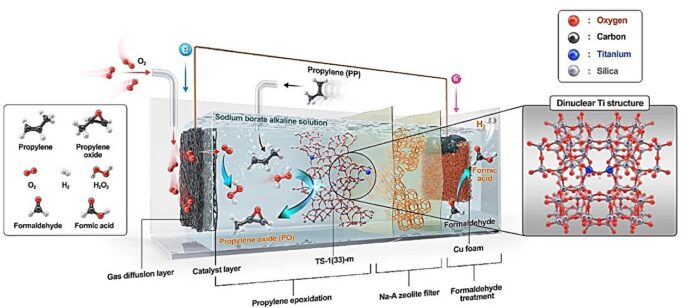
A new, environmentally friendly process for producing propylene oxide (PO) – a crucial ingredient in everyday items like sofas, mattresses, and water bottles – has been developed. What makes this innovation stand out is its ability to operate without external energy sources like electricity or sunlight.
The Problem with Traditional PO Production
Propylene oxide is produced through the oxidation of propylene. Historically, this process relied on hydrogen peroxide (H₂O₂), typically sourced from the anthraquinone process. This method is problematic because it depends heavily on fossil fuels and generates substantial carbon dioxide (CO₂) emissions—contributing to climate change.
A Breakthrough: In-House H₂O₂ Generation
Researchers led by Professors Ja Hun Kwak and Ji-Wook Jang from UNIST, alongside Professor Sung June Cho of Chonnam National University, have created a self-driven system capable of producing PO using in-house generated hydrogen peroxide. Here’s how it works:
- Autonomous H₂O₂ Creation: The system generates H₂O₂ through an electrochemical reaction involving oxygen and formaldehyde. This reaction is driven by a chemical potential—the energy difference between the reactants and products—allowing the system to operate spontaneously, without external power.
- Integrated PO Synthesis: The generated H₂O₂ then reacts with propylene within the system, directly synthesizing propylene oxide.
- Catalyst Innovation: The team significantly improved the catalyst’s structure, overcoming a limitation of conventional zeolite catalysts (TS-1) which become less effective in alkaline environments. This advancement increases the efficiency of propylene oxidation and boosts PO yields.
Performance and Economic Advantages
The new system demonstrates impressive performance:
- High Output: Over 24 hours, the system produced 1,657 micromoles (μmol) of PO per square centimeter (cm²), approximately eight times higher than previous eco-friendly H₂O₂-based production methods.
- Clean Energy Co-Product: The process can also simultaneously produce hydrogen (H₂), a clean and valuable energy source.
- Reduced Costs: Economic analysis suggests this new system can reduce PO production costs by about 8%, bringing the price down to around $2.168 per kilogram—a competitive advantage over traditional methods.
Decentralized and Accessible Production
Beyond cost savings, the system offers significant operational benefits:
- Simplified Design: It eliminates the need for complex pre-treatment steps and energy-intensive high-temperature, high-pressure equipment.
- On-Site Generation: Producing H₂O₂ on-site minimizes transportation and storage costs and logistical challenges.
- Scalability and Flexibility: The modular design allows for easy installation at various locations, facilitating small-scale, customized production and promoting a shift away from centralized manufacturing towards more distributed systems.
“This modular process can be easily installed at various sites, enabling small-scale, customized production and promoting a shift from centralized large-scale manufacturing to decentralized, distributed systems,” says Professor Jang.
“This work represents a significant step forward in overcoming the long-standing limitations of zeolite catalysts, paving the way for a much more sustainable and environmentally friendly chemical industry,” adds Professor Kwak.
This innovation represents a significant step towards a more sustainable chemical industry, potentially revolutionizing how essential plastic ingredients are produced by making the process more efficient, cost-effective, and environmentally responsible. It highlights the potential of harnessing chemical potential to drive sustainable industrial processes.